THE EUROPEAN COLLABORATIVE ACTION ON MEDICATION ERRORS & TRACEABILITY
“All medication errors are potentially preventable. They can be reduced or avoided by improving systems and practices in medication, including purchasing, prescribing, preparation, dispensing, administration and monitoring”
– World Health Organisation (WHO) 2017 (1)
MEDICATION AND PATIENT HARM
The United States National Coordinating Council for Medication Error Reporting and Prevention defines a medication error as: “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer. Such events may be related to professional practice, health care products, procedures, and systems, including prescribing, order communication, product labelling, packaging, and nomenclature, compounding, dispensing, distribution, administration, education, monitoring, and use”.(2)
Medication errors are a common cause of harm to patients in acute care settings. They cause the highest adverse events in hospitals, not only in terms of number, but as well in morbidity and mortality.(3)
It has been estimated that in some countries approximately 6-7% of hospital admissions appear to be medication related, with over two-thirds of these considered avoidable and therefore due to errors. These errors can lead to no harm, may be minor or range to major errors which can result in morbidity, mortality and poor quality of life for the patient. In turn, these may have significant health and economic consequences, including the increased use of health services, preventable medication-related hospital admissions and death.
Medication errors can include prescribing, preparation, dispensing, administration mistakes as well as monitoring errors.
According to the WHO, medication errors occur when unreliable medication systems and/or human factors such as fatigue and lack of health care workers affect the practice of prescribing, dispensing, administering and monitoring medication. The most frequent errors occur during the medication administration phase in hospitals. High workloads and lack of health care personnel contribute to 23% of medication errors.(1)
Despite the lack of European data, there is a high variability across the 27 Member States in terms of risk of suffering one medication error in acute care settings. Significant imbalances in terms of health care professionals quality and quantity (in particular pharmacy and nursing profession) and IT infrastructure are the main drivers of variability.
6 REASONS TO ACT NOW
1. Medication errors in the hospital setting represent the most common adverse effect on health systems
Medication errors constitute the highest adverse events in hospitals, not only in terms of number, but as well in morbidity and mortality.
Despite the lack of consolidated data of medication errors at the European Union level, according to the European Medicines Agency the medication-error rate in the hospital setting varies from between 0.3% and 9.1% at prescription initiation and between 1.6% and 2.1% at the dispensing stage.(4)
In the UK, a 2017 study in the English NHS(5) quantified 237 million medication errors in one year, 21.3% in prescription, 15.9% in dispensing and 54.4% in administration.
In Spain, the National Study on Adverse Effects Linked to Hospitalisation (ENEAS 2005)(6) published in 2006 concluded that the incidence of adverse effects in hospitalised patients was 8.4%, the most common adverse effect being medication errors, which accounted for 37.4% of the total. The “Patient Safety Strategy in the National Health System. 2015-2020 period”(7) indicates that there are up to 17 medication incidents per day for every 100 patients hospitalised, 16% in prescription, 27% in transcription, 48% in dispensing and 9% in administration.
2. Medication errors in a hospital setting are preventable
According to the WHO, all medication errors are potentially preventable.(1)
According to the ENEAS study in Spain, 42.8% of adverse effects are deemed preventable.(6)
Clinical evidence shows that the introduction of traceability systems in hospitals would allow a significant reduction in medication errors, as well as improve the efficiency and quality of care of nursing staff.
3. Medication errors cause suffering for patients and incremental costs for health systems, which undermine their sustainability
Every day, medication errors in the health care field lead to unnecessary lengthening of stays for patients with an enormous additional cost for health care systems, patients and their families. They also involve indirect social costs, resulting from the loss of productivity due to disability.
The WHO estimates the annual cost of medication errors at $42 billion USD annually.(1)
In Spain, the “Patient Safety Strategy in the National Health System. 2015-2020 period”(7) estimates the cost of medication errors around 2 billion euros (representing 3% of the total National Healthcare expenditure).
The estimated cost to the NHS of avoidable adverse events related to medication in hospitalised patients, plus those that led to hospital admissions and emergency consultations, would be approximately £98.5 million (representing 2.9% of NHS health care expenditure).(5)
4. Preventing medication errors in the hospital setting is a good practice that promotes the well-being of everybody
The prevention of medication errors is a requirement for safe and quality health care, and constitutes best practice that satisfies bioethical requirements.(9)
5. Global priority launched by the WHO in 2017
A global initiative called “The Third Global Patient Safety Challenge: Medication Without Harm”(1) aims to reduce medication errors and the associated harm in all countries around the world by 50% within 5 years. In this third challenge, health ministers are invited to establish national plans covering four aspects of the safe use of medication: the involvement of patients and the general public; medicines as products; the education, training and monitoring of health professionals; and medication management systems and practices.
6. The solution is available and affordable
There is no doubt that education of health care professionals combined with technology and reporting processes are critical success factors in solving this patient safety issue.
Nowadays, the level of technology enables systems that guarantee the traceability of medication in hospitals to prevent errors, including, among others: automated storage and electronic dispensing cabinets systems, electronic prescription (CPOE) systems, electronic preparation systems, bar-coded single-dose systems, barcoded medication administration systems (BCMA) and smart medication administration pumps, connected if possible to BCMA and prescription systems, resulting in:
- A reduction in the number of errors in the prescription phase of medicines by preventing errors arising from manual prescriptions (errors in writing, reading and interpretation errors, etc.).
- A reduction in the number of errors in the preparation phase. Each step in the preparation phase is traced and the process is stopped if an error is detected. The medication bar-code scanning system, during preparation of medication, mainly in wards, prevents errors due to the iso-appearance of the medications, which contribute to 33% of medication errors(3), among other potential causes.
- The identification of the patient and the medication prior to the administration of any medication (BCMA), especially intravenous, eliminates administration errors due to the wrong patient, the wrong medication and the wrong route.
- Reduction in manual documentation and the number of steps required due to the fact that manual processes are automated. It is estimated that up to 40% of nursing time is spent on administrative and non-health care activities.(9)
In short, medication traceability systems make it possible to standardise hospital processes, prevent medication errors in all phases of the process and improve nursing efficiency, allowing more time to be spent on care and less time on administrative tasks.
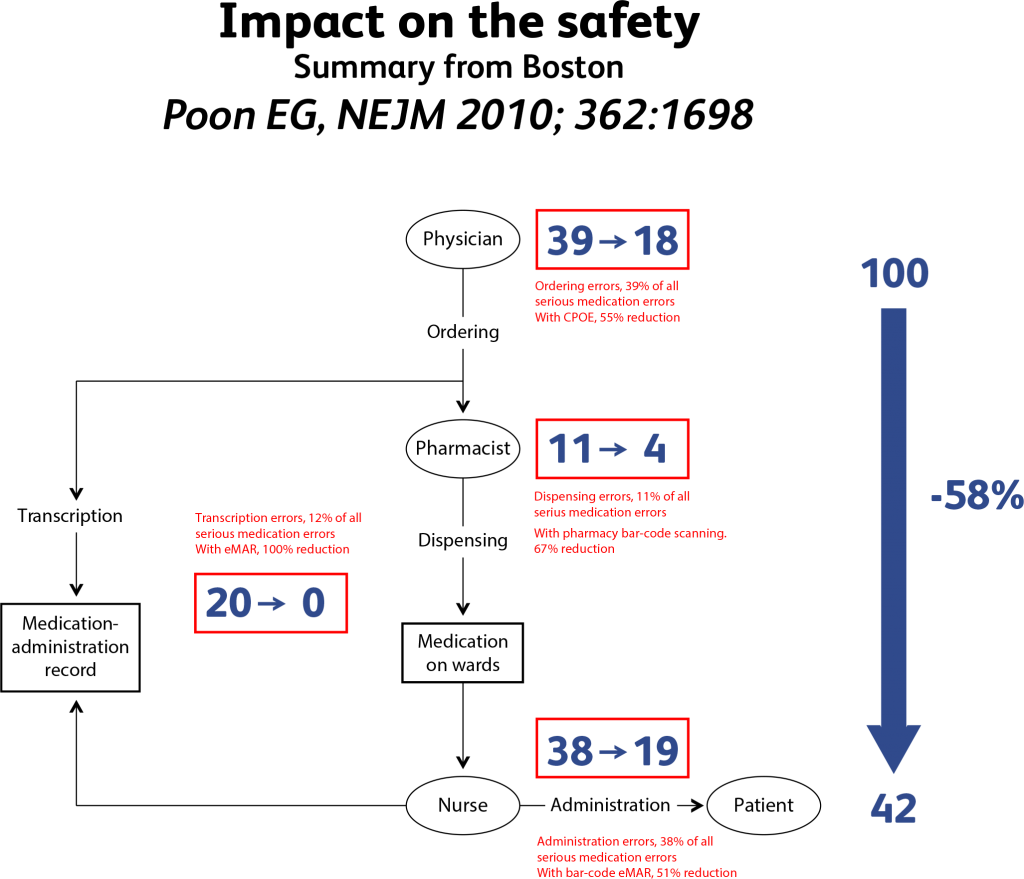
The summary of the studies published in Boston(10) (see table 1 below) show that IT can reduce medication errors by around 60%. It clearly demonstrates that the introduction of IT systems has a very significant impact, but that other actions, such as clinical pharmacy, patient education, drug packages design, etc have to be applied.
THE EUROPEAN COLLABORATIVE ACTION ON MEDICATION ERRORS AND TRACEABILITY
PROJECT’S OVERVIEW
The European Alliance for Access to Safe Medicines (EAASM) is an independent, pan-European initiative dedicated to protecting patient safety by ensuring access to safe and legitimate medicines. The EAASM champions patient safety initiatives relating to medication safety, such as compounding practices, unlicensed/off-label usage of medicines and the field of nanomedicines to help ensure patient safety and wellbeing across Europe.
As a result of the burden of medication errors in acute care setting across European countries, the EAASM board has decided to launch a major European patient safety project aimed to promote the prevention of medication errors in acute care settings through the implementation of medication traceability systems.
To achieve the objective of the project , the EAASM is working to create one European alliance made up of Scientific and Patients’ organisations, under the project name “The European Collaborative Action on Medication Errors and Traceability” [ECAMET].
With patient safety at its heart, the Alliance’s composition and focus will include experts from acute care settings that are more susceptible to medication errors namely: pharmacy, oncology and intensive care, nurses as well as patient organisations.
Key initiatives will be focused on ensuring medication errors are prevented through awareness, education and promotion of traceability systems in acute care settings across Europe.
PROJECT’S OBJECTIVES
The project has the overall objective to reduce medication errors and promote, at European and national levels, the implementation of comprehensive electronic traceability systems in acute care settings, thus enhancing patient safety and quality of healthcare:
1. Create awareness of the importance of medication patient safety in the hospital environment
2. Call on European institutions and Member State’s authorities to promote regulations and guidelines on medication traceability to prevent medication errors.
3. Promote medication traceability in European Member States with a focus on acute care settings as the most efficient way to prevent medication errors.
Key outputs:
- Improve awareness and education on medication errors among clinicians and patients in Europe.
- Improve national surveillance systems on medication errors in acute care settings to measure and monitor the evolution of the burden and its reduction.
- Promote the role of medication traceability systems (see Annex I) across European countries as the most effective way to prevent medication errors in acute care settings.
- Reduce manual documentation and administrative burden for health care workers to enable more time for clinical value added activities.
- This will result in a significant reduction in the number of medication errors across European countries in acute care settings.
PROJECT’S STRATEGY AND TACTICS
To achieve the objectives of the ECAMET project , the following strategic initiatives will be taken:
1. Creation of a European Alliance of Scientific and Patient organisations
The EAASM is contacting European Scientific and Patient organisations to be part of the ECAMET project. The following associations have positively welcomed and endorsed the ECAMET project:
- UEHP – European Union of Private Hospitals
- EHMA – European Health Managers Association
- ESNO – European Specialist Nurses Organisation
- ESOP – European Society of Oncology Pharmacists
- SEFH – Spanish Society of Hospital Pharmacists
- ESICM – European Society of Intensive Care Medicine
- ESPNIC – European Society of Paediatric and Neonatal Intensive Care
- EUPSF – European Patient Safety Foundation
- EPF – European Patients’ Forum (TBC)
- FEP – Spanish Patients’ Forum(8)
- HFE – Health First Europe
- MPNE – Melanoma Patient Network Europe
- ISMP – Spain – Institute for Safe Medication Practices – Spain
- ECO – Fundación para la Excelencia y la Calidad de la Oncología
2. Creation of a Scientific Committee (SC)
The SC will oversee the project and ensure that each step will be scrutinised and thus optimise the outcomes. The SC will include representatives of organisations, scientific associations and experts, who will drive the ECAMET project forward and generate awareness of the topic.
The Scientific Committee currently comprises:
- Clinical oncologist
- Hospital pharmacists
- Clinical intensive care specialist for adults
- Clinical intensive care specialist for paediatrics
- Patients’ groups representative
- Specialist nurse
- Private hospital representative
- Medication safety representative
- Patient Network representative
- EAASM representative
3. Creation of a comprehensive Pan-European survey among clinicians
The Pan-European survey among clinicians will be managed by a European market research company.
It will include questions about the size of the problem of medication errors, the level of awareness and education and the existing traceability systems in place in acute care settings.
The survey will be delivered to the following groups of clinical experts: oncologists, pharmacists, intensive care specialists and nurses.
The pan European survey will be conducted in large countries and one small to medium size country and the methodology will ensure a statistically representative sample.
There will be a consolidated report and separate reports by specialty namely: General, Oncology and Intensive Care Units.
4. Creation of a White Paper
The Pan-European survey will act as the foundation for a White Paper which highlights the issues surrounding medication errors across several EU countries and puts forward solutions and opportunities for the future to prevent them
5. Creation of a Joint Call to Action
Creation of a Joint Call to Action calling upon health authorities, policy makers, health care professionals and patients to join hands to prevent unnecessary harm in hospital settings by promoting medication traceability and innovative quality standards for patient safety across Europe.
6. Advocacy work
Advocacy work at institutional level (Europe and country state members), including bilateral meetings with relevant policy makers of the European Parliament and European Commission to build momentum and gather consensus, along with an awareness-raising campaign at European level. Advocacy work will include:
- A roundtable in the EU Parliament to launch the White Paper and the Joint Call to Action.
- Dissemination of the Joint Call to Action at country state level through round tables and awareness campaigns.
- Dissemination of best practices in acute care settings in Europe.
PROJECT’S TIMELINE
- Summer 2019 – Spring 2020: Mapping and creation of the ECAMET Alliance
- Summer 2020: Creation of the Scientific Committee and kick-off meeting
- Autumn 2020: Pan-European Survey
- Spring 2021: Survey report and Joint Call to Action on Medication Errors and Traceability
- Spring/Summer 2021: Awareness campaign and evidence building
- Winter 2021: Parliament meeting and advocacy work at institutional level. Dissemination of best practices in Europe
Governance
Stakeholders participating in the ECAMET are voluntary participants and therefore cannot be required to append their names to documents, publications, etc without their consent. The European Alliance for Access to Safe Medicines (EAASM) would oversee the project stages in collaboration with the members of the Scientific Committee and will be responsible for progressing the deliverables of the ECAMET.
The EAASM is an independent non profit, pan-European initiative dedicated to protecting patient safety by ensuring access to safe and legitimate medicines.
BIBLIOGRAPHY
Ref 1 World Health Organisation. Patient safety. The third WHO Global Patient Safety Challenge: Medication Without Harm. [On line] 2017. https://www.who.int/patientsafety/medication- safety/medication-without-harm-brochure/en/
Ref 2 https://www.nccmerp.org/about-medication-errors
Ref 3 Elliott R, Camacho E, Campbell F, Jankovic D, Martyn St James M, Kaltenthaler E, Wong R, Sculpher M, Faria R, (2018). Prevalence and Economic Burden of Medication Errors in The NHS in England. Rapid evidence synthesis and economic analysis of the prevalence and burden of medication error in the UK. Policy Research Unit in Economic Evaluation of Health and Care Interventions. Universities of Sheffield and York.
Ref 4 5EMA 2013 https://www.ema.europa.eu/en/news/tackling-medication-errors-european-medicines-agency-workshop-calls-coordinated-eu-approach.
Ref 5 National Health System (NHS). NHS Improvement. [On line] 2017. https://improvement.nhs.uk/resources/national-medicines-safety-programme/.
Ref 6 Ministry of Health and Consumption. Estudio Nacional sobre los Efectos Adversos ligados a la Hospitalización [National Study on Adverse Effects associated with Hospitalisation]. ENEAS 2005. Madrid: s.n., 2006. https://www.seguridaddelpaciente.es/resources/contenidos/castellano/2006/ENEAS.pdf.
Ref 7 Ministry of Health, Social Services and Equality. Patient Safety Strategy in the National Health System 2015-2020. [On line] 2016. https://www.seguridaddelpaciente.es/resources/documentos/2015/Estrategia%20Seguridad%20del%2 0Paciente%202015-2020.pdf.
Ref 8 Alianza multidisciplinar frente a los errores en la medicación del paciente hospitalizado en España como buena practica científica [Multi-disciplinary alliance against errors in hospitalised patients in Spain as good scientific practice] Herranz, A, and others. Madrid: Carlos III Health Institute, 2019. V Congress on Bioethics.
Ref 9 Estimación de necesidad de personal de enfermería de una unidad; cálculos prácticos [Estimate of the need for nursing staff in a unit; practical calculations]. Fenandez Diez, A. Madrid: Escuela Nacional de Sanidad [National School of Health] 2013, Vol. Topic 10.6. http://e- spacio.uned.es/fez/eserv/bibliuned:500713/n10.6_necesidad_de_personal_de_enfermer a.pdf.
Ref 10 Eric G. Poon, M.D., M.P.H., Carol A. Keohane, B.S.N., R.N., Catherine S. Yoon, M.S., Matthew Ditmore, B.A., Anne Bane, R.N., M.S.N., Osnat Levtzion-Korach, M.D., M.H.A., Thomas Moniz, Pharm.D., Jeffrey M. Rothschild, M.D., M.P.H., Allen B. Kachalia, M.D., J.D., Judy Hayes, R.N., M.S.N., William W. Churchill, M.S., R.Ph., Stuart Lipsitz, Sc.D., Anthony D. Whittemore, M.D., David W. Bates, M.D., and Tejal K. Gandhi, M.D., M.P.H. N Engl J – Med 2010; 362:1698-1707, DOI: 10.1056/ NEJMsa0907115
Ref 11 Perras C, Jacobs P, Boucher M, Murphy G, Hope J, Lefebvre P, et al. Technologies to reduce errors in dispensing and administration of medication in hospitals: clinical and economic analyses (Structured abstract). Health Technology Assessment Database. 2016 Karavasiliadou, S & Athanasakis, E. An inside look into the factors contributing to medication errors in the clinical nursing practice. Health Science Journal. Volume 8 (2014) Issue http://www.hsj.gr/medicine/an-inside-look-into-the-factors-contributing-to-medication-errors-in-the-clinical-nursing-practice.pdf Hassink J, Jansen M, Helmons P. Effects of bar code-assisted medication administration (BCMA) on frequency, type and severity of medication administration errors: a review of the literature. European Journal of Hospital Pharmacy. 2012;16:489-94.
Ref 12 National Patient Safety Agency (NPSA). Safety in doses: medication safety incidences in the NHS. 2007. http://www.nrls.npsa.nhs.uk/EasySiteWeb/ getresource.axd?AssetID=61392 Karavasiliadou, S & Athanasakis, E. An inside look into the factors contributing to medication errors in the clinical nursing practice. Health Science Journal. Volume 8 (2014) Issue 1. http://www.hsj.gr/medicine/an-inside-look-into-the-factors-contributing-to-medication-errors-in-the-clinical-nursing-practice.pdf
Ref 13 Anonymous. ASHP guidelines on preventing medication errors with antineoplastic agents. American Journal of Health-System Pharmacy. 2002;59(17):1648-68.
Ref 14 Karavasiliadou, S & Athanasakis, E. An inside look into the factors contributing to medication errors in the clinical nursing practice. Health Science Journal. Volume 8 (2014) Issue 1. http://www.hsj.gr/medicine/an-inside-look-into-the-factors-contributing-to-medication-errors-in-the-clinical-nursing-practice.pdf
ANNEX 1
THE ROLE OF MEDICATION TRACEABILITY
The role of medication traceability in preventing such errors in acute care settings is critical. Medication traceability includes the following concepts:
- Automated storage and electronic dispensing cabinets systems
- e-Prescription and e-preparation systems
- Electronic scanning systems, such as barcoded medication administration (BCMA)
- Smart pumps
- Full connectivity of systems in the acute care setting
Studies have shown that Medication traceability technology results in the following benefits.(11)
- Being able to retrieve a patient back into the health care system who has received a substandard drug (batch recall of a falsified medicine).
- Reduction in the number of medication and human errors in medication prescription.
- Reduction in medication preparation errors. Medication steps are tracked and the process is stopped if an error is detected. The scanning system double checks the information and sends an alert that something is amiss. Lookalike, soundalike medication contributes to 33% of administration errors.(12,13)
- Acts as a double check for the nurse. The recommended best practice standard is for two nurses to check IV high-risk medications prior to administration (the four eyes principle) to reduce human error. Barcoded Medication Administration (BCMA) reduces the staffing burden for a second physical check and frees up nursing resource. High workloads and low staffing contribute to 23% of medication administration errors.(14)
- Reduces distractions. If a nurse is not interrupted to perform a second check on IV medications then there are less distractions. Distractions (e.g. being pulled away, doing two things at once) contribute to medication errors, resulting in an improper “check”.(15)
- Reduces manual documentation. After each administration, nurses have to physically document the time, date and the name of the nurse who administers it. With medication traceability systems this can be done automatically. This applies to invoices processing as well.
- Reduces the number of steps required, as manual steps are automated. Up to 40% of nursing time is spent on administrative tasks (such as documentation) instead of clinical activities.
- BMCA has the ability to track and trace the entire medication journey, such as alerts to the wrong location.
- If an infusion pump is included in this administration process, BCMA can be used to check administration rates are correctly programmed.
- Reduce medication dispensing errors to inpatients and outpatients alike.
- Reduce costs and inefficiencies in managing medication inventory and optimise stocks.
- Better track and trace on medication shortages and use of alternative medications

To find out more about the EAASM, visit www.eaasm.eu
Please contact EAASM Director Mike Isles
mike.isles@eaasm.eu
EU Transparency Register Number: 861368611058-84
